Types of RSV Immunizations
RSV Vaccines to Protect Adults 60+
Based on shared clinical decision making (discussions between the patient and health care provider), adults 60 years and older can receive a single dose of RSV vaccine.
There are 2 RSV vaccines licensed by the U.S. Food and Drug Administration for use in adults 60 years and older:
- Arexvy (RSVPreF3 vaccine)
- Abrysvo (RSVpreF vaccine)
Adults 60 years and older have an increased risk of developing serious health complications from RSV because immune systems weaken with age. In addition, certain underlying medical conditions may increase the risk of getting very sick from RSV and older adults with these conditions may especially benefit from getting the RSV vaccine.
If you are 60 years and older, talk to your healthcare provider to see if the RSV vaccine is right for you. RSV vaccine can be given at the same time as other vaccines such as flu and COVID-19 vaccines.
RSV Immunizations to Protect Infants 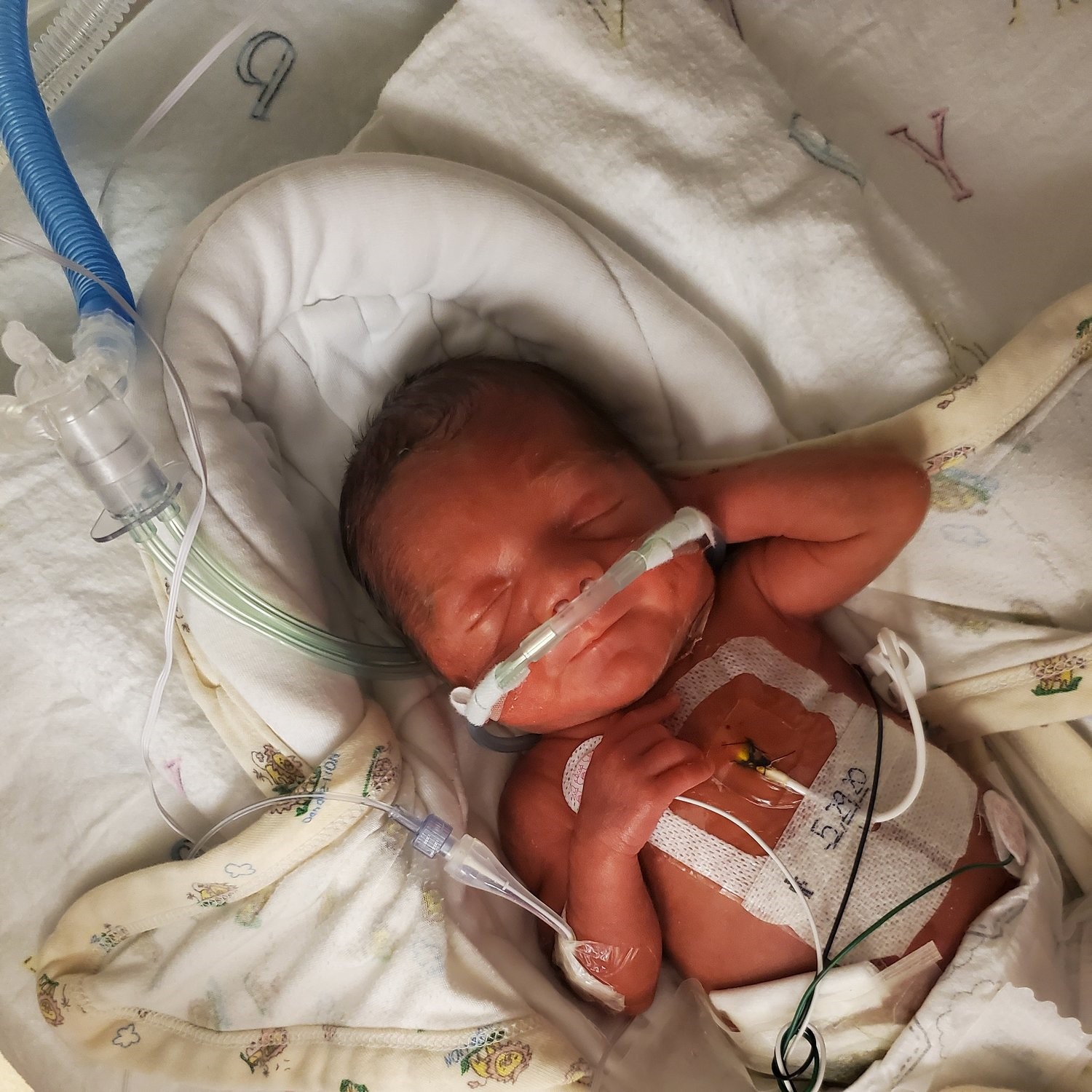
There are two ways to protect babies from getting very sick with RSV. One is an RSV vaccine, called Abrysvo, given to the mother during pregnancy. The other is an RSV immunization, called Nirsevimab, that gives protective antibodies directly to an infant after birth to help their immune system fight off severe illness caused by RSV.
- RSV vaccine (Abrysvo) given to pregnant people who are 32-36 weeks pregnant during RSV season (September-January) to protect their baby from RSV after birth.
- Infants younger than 8 months of age, born during- or entering- their first RSV season should get one dose of Nirsevimab (RSV Immunization) within 1 week after birth. A dose of RSV immunization is also recommended for children between the ages of 8 and 19 months entering- their second RSV season who are in at least one of these groups:
- Children who have chronic lung disease from being born prematurely
- Children who are severely immunocompromised
- Children with cystic fibrosis who have severe disease
- American Indian and Alaska Native children
If you receive an RSV vaccine at least 2 weeks before delivery, your baby will have protection and, in most cases, should not need an RSV immunization after birth. However, there may be some situations in which Nirsevimab (RSV immunization) would be recommended for an infant after the mother received an RSV vaccine.
Risks of RSV Immunizations
Side effects after vaccination are common and typically go away in a few days.
- Pain, redness, and swelling where the shot is given, fatigue (feeling tired), fever, headache, nausea, diarrhea and muscle or joint pain.
Adverse events (serious health problems or allergic reactions) are rare. If you see signs of a severe allergic reaction (hives, swelling of the face and throat, difficulty breathing, a fast heartbeat, dizziness, or weakness), call 9-1-1 and get the person to the nearest hospital.
For other signs that concern you, call your health care provider.
Risks of RSV Vaccine for Adults 60+
Risks of RSV Vaccine for People who are Pregnant
Risks of RSV Immunization for Infants
Last Reviewed: January 2, 2024

